Nuclear bonds - hydrogen
bonds.
Hydrogen is not an indestructible atom, it is a vector space condensed
into
orthogonally closed vector circuits, empirically known, electromagnetic.
The interaction of vector properties constitutes vector energy.
The centripetal forces in orthogonally closed vector circuits compress
each
other and compose a microscopic, solid, cold structure - hydrogen.
The interactions of vector properties compose and decompose vector structures.
Hydrogen structures form bonds between themselves when they enter into
oscillations, closed circuits break and open, vector polarities of bonding.
Each circuit thus acquires a positive and a negative polarity, four in
total.
The polarities of hydrogen structures polarize vector space, close circuits
at a distance, and their centripetal forces attract structures, like magnets.
Obviously, the closed bonds become orthogonally closed vector circuits,
force fields, vector space with high orientation density in direction
and sense.
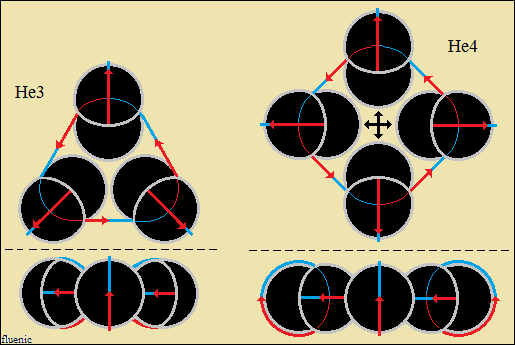
Thus, bonds are formed between two, three and four hydrogen structures,
each connected with orthogonally closed vector circuits, deuterium and
helium.
The forces that hold the structures together are vector forces of attraction,
centripetal forces, stronger than central forces of repulsion. The bonds
between two, three and four hydrogen structures, orthogonally closed
vector circuits, "electromagnetic", have centripetal vector
forces
(not gravity) and central forces of repulsion, as in macroscopic structures.
Repulsive forces are the parallel vector circuits, "the magnetic
axis".
The balance between these forces explains the specific reactions,
fusions and fissions (radioactivity) characteristic of these structures.
The conditions for these reactions exist only in the photosphere of stars.